15 years one-stop China custom CNC machining parts factory
 417 |
Published by VMT at Dec 26 2024 | Reading Time:About 8 minutes
417 |
Published by VMT at Dec 26 2024 | Reading Time:About 8 minutes
Are you facing challenges in enhancing the durability and aesthetic appeal of your steel CNC machining parts, unsure if anodizing is a viable solution? Many manufacturers grapple with the decision of whether to anodize steel, considering factors like cost, process complexity, and desired outcomes. This uncertainty can lead to suboptimal product performance, increased maintenance costs, and missed opportunities for innovation. Fortunately, understanding the intricacies of steel anodizing can provide the clarity needed to make informed decisions. By exploring the anodizing process, its advantages, and viable alternatives, you can optimize your CNC machining services to produce superior, cost-effective, and high-quality steel anodized CNC machining parts. This comprehensive guide delves into whether steel can be anodized, the benefits it offers, and the best alternatives available, empowering your CNC machining factory to achieve excellence in custom CNC machining.
Steel can be anodized through specialized processes that enhance its corrosion resistance and surface hardness. While more complex and costly than anodizing aluminum, steel anodizing offers improved durability and aesthetic finishes. Understanding the process and benefits of steel anodizing helps manufacturers decide whether it suits their CNC machining needs.
To determine if anodizing steel is the right choice for your CNC machining projects, it's essential to first grasp the fundamentals of anodizing and why this process is applied to metals. This foundational knowledge will pave the way for a deeper exploration into the feasibility, challenges, and specific procedures involved in anodizing steel. Additionally, understanding the advantages and limitations of steel anodizing will help you evaluate its suitability compared to alternative surface treatment methods, ensuring that your CNC machining services deliver optimal results.
Foreword
In the competitive landscape of CNC machining parts manufacturing, surface treatment plays a crucial role in enhancing the performance, durability, and aesthetic appeal of metal components. Anodizing, a well-established electrochemical process, is widely recognized for its ability to improve the surface properties of metals, particularly aluminum. However, the question arises: Can steel be anodized with similar efficacy and benefits? This article aims to provide a comprehensive exploration of steel anodizing, detailing the processes involved, the advantages it offers, the challenges faced, and the viable alternatives available. By delving into the specifics of steel anodizing, manufacturers can make informed decisions on whether this surface treatment aligns with their production goals and customer requirements, ultimately enhancing the quality and value of their CNC machined parts.
Anodizing is an electrochemical process that enhances the natural oxide layer on the surface of metals, primarily aluminum and titanium, to increase their corrosion resistance, surface hardness, and aesthetic appeal. The process involves immersing the metal in an electrolyte solution and applying an electric current, which causes oxygen ions to bond with the metal surface, forming a stable and durable oxide layer. This oxide layer is integral to the metal, meaning it cannot peel or chip off, unlike paints or coatings.
Why Anodize Metals?
The primary reasons for anodizing metals are to improve their durability, enhance corrosion resistance, and provide a better surface for dyeing or painting. Anodizing also increases the surface hardness, making the metal more resistant to wear and abrasion. Additionally, anodized surfaces can achieve a variety of colors through dyeing, offering aesthetic versatility for different applications.
In the context of CNC machining parts manufacturing, anodizing serves multiple purposes. It not only protects the machined components from environmental factors that could lead to corrosion and degradation but also improves their appearance, making them more attractive for consumer-facing applications. Furthermore, the enhanced surface hardness from anodizing can extend the lifespan of the parts by reducing wear and tear during use. Overall, anodizing is a valuable surface treatment that adds both functional and aesthetic value to metal components.

Anodizing steel is indeed feasible, but it is more challenging and less common compared to anodizing aluminum. While aluminum readily forms a stable and protective oxide layer through anodizing, steel requires more specialized processes to achieve similar results. The feasibility of steel anodizing depends on several factors, including the type of steel alloy, the desired thickness and properties of the oxide layer, and the specific application requirements.
Types of Steel Suitable for Anodizing
Not all steel alloys are suitable for anodizing. Generally, stainless steels, particularly those with high chromium content, are more amenable to anodizing due to their enhanced corrosion resistance and stable oxide layers. Carbon steels and other less alloyed steels can also be anodized but may require additional surface preparation and more controlled anodizing conditions to achieve the desired results.
Process Adaptations for Steel Anodizing
Anodizing steel often involves higher voltages and more stringent control of process parameters compared to aluminum. The electrolyte solutions used, typically sulfuric acid or other specialized chemicals, need to be carefully managed to ensure uniform oxide layer formation. Additionally, pre-treatment steps such as cleaning, surface roughening, and etching are critical to remove impurities and prepare the steel surface for effective anodizing.
Challenges in Steel Anodizing
The anodizing process for steel is inherently more complex and cost-intensive. Steel's composition, particularly its higher carbon content and potential for hydrogen embrittlement, necessitates precise process control to prevent defects in the oxide layer. Moreover, achieving consistent thickness and uniformity of the anodized layer on steel parts can be more difficult, impacting both the protective and aesthetic qualities of the anodized surface.
Despite these challenges, advancements in anodizing technology and a deeper understanding of steel's electrochemical behavior have made steel anodizing more achievable. Manufacturers who invest in specialized equipment and expertise can successfully anodize steel, thereby enhancing the performance and longevity of their CNC machined parts.
Galvanic corrosion is a significant concern in the anodizing of steel, particularly when the anodized steel is in contact with other metals. This type of corrosion occurs when two different metals are electrically connected in the presence of an electrolyte, leading to the more anodic metal corroding faster than the cathodic metal.
Understanding Galvanic Corrosion
In the context of anodized steel, galvanic corrosion can undermine the protective benefits provided by the anodized layer. When anodized steel is paired with a more noble metal, such as copper or stainless steel, the steel can act as the anode and corrode preferentially. This is especially problematic in environments where moisture or corrosive agents are present, as the electrolyte (water containing ions) facilitates the galvanic reaction.
Impact on Anodized Steel Components
The presence of galvanic corrosion can lead to localized pitting, loss of material, and compromised structural integrity of anodized steel components. Over time, this can result in the degradation of both the anodized layer and the underlying steel, reducing the overall lifespan and reliability of the CNC machined parts.
Preventing Galvanic Corrosion
To mitigate the risks of galvanic corrosion in anodized steel, several strategies can be employed:
1. Material Selection: Choosing metals with similar electrochemical potentials can minimize the galvanic potential difference, reducing the likelihood of galvanic corrosion.
2. Insulation: Physically separating anodized steel from other metals using insulating materials such as gaskets, coatings, or plastic barriers can prevent electrical contact and thus galvanic reactions.
3. Environment Control: Reducing the presence of electrolytes by controlling humidity levels and avoiding exposure to corrosive substances can limit the conditions necessary for galvanic corrosion to occur.
4. Anodizing Thickness: Increasing the thickness of the anodized layer can provide additional protection against corrosion, making it more difficult for the galvanic reaction to penetrate and affect the underlying steel.
5. Sealants and Coatings: Applying sealants or additional protective coatings on anodized steel can further enhance its corrosion resistance and act as a barrier to galvanic interactions.
By implementing these preventive measures, manufacturers can significantly reduce the risks associated with galvanic corrosion, ensuring that anodized steel components maintain their integrity and performance over extended periods.
Preparation for Anodizing
Successful anodizing of steel requires meticulous preparation to ensure that the surface is free of contaminants and properly conditioned for oxide layer formation. The preparation phase typically involves several key steps:
Surface Cleaning
Thorough surface cleaning is essential to remove oils, greases, dirt, and other contaminants that can interfere with the anodizing process. This is typically achieved through a combination of chemical cleaning agents, such as degreasers and detergents, and mechanical cleaning methods like abrasive blasting or ultrasonic cleaning. Ensuring a pristine surface is crucial for achieving a uniform and adherent anodized layer on the steel.
Surface Roughening (Optional)
Surface roughening may be employed to enhance the adhesion of the anodized layer and improve its mechanical interlocking with the steel substrate. This can be achieved through mechanical methods, such as sandblasting or grinding, or chemical etching using acids. A roughened surface provides more surface area and micro-porosities for the oxide layer to bond with, increasing the durability and corrosion resistance of the anodized steel.
Surface Etching (Optional)
Surface etching involves using chemical solutions to uniformly remove a thin layer of the steel surface, creating a smooth and reactive surface for anodizing. This step is particularly important for steel alloys that are prone to uneven oxide layer formation. Etching helps in achieving a consistent surface texture, which is critical for the uniformity and effectiveness of the anodized layer.
The anodizing procedure for steel involves several carefully controlled steps to ensure the formation of a protective and aesthetically pleasing oxide layer. This process is more intricate than anodizing aluminum, requiring precise control over various parameters to achieve the desired results.
Pretreatment
Pretreatment is a critical phase that involves cleaning and conditioning the steel surface to prepare it for anodizing. This step typically includes degreasing to remove organic contaminants, followed by etching or roughening to create a surface texture conducive to oxide layer formation. The goal of pretreatment is to ensure that the anodizing process can proceed without interruptions caused by surface impurities or irregularities.
Setting up the Electrolytic Cell
Setting up the electrolytic cell involves assembling the anodizing bath, which typically consists of an electrolyte solution, electrodes, and the steel workpieces. The electrolyte used can vary depending on the desired properties of the anodized layer but often includes acids such as sulfuric acid or oxalic acid. Proper configuration of the cell is essential to maintain consistent electrical conditions and ensure uniform oxide layer growth across the steel surfaces.
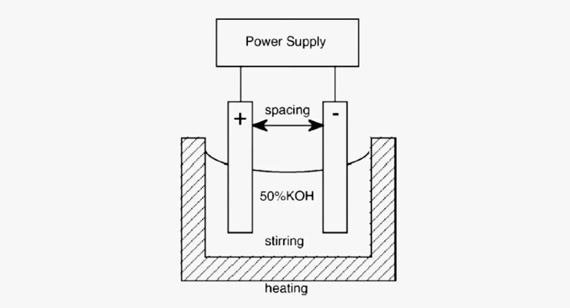
Alkaline Solution Concentration and Composition
The concentration and composition of the alkaline solution play a significant role in the anodizing process. Adjusting these parameters allows for control over the thickness and properties of the oxide layer. Higher concentrations of alkaline agents can increase the rate of oxide layer formation, while specific additives may be included to enhance certain characteristics such as hardness or corrosion resistance. Precise control of the solution’s chemistry is necessary to achieve the desired outcome.
Voltage
Applying the correct voltage is crucial for driving the anodizing reaction. The voltage must be carefully regulated to ensure that the steel is anodized uniformly without causing excessive heating or damaging the oxide layer. The optimal voltage range varies depending on the type of steel alloy and the specific anodizing parameters being used. Consistent voltage application helps in achieving a stable and uniform oxide layer that provides effective protection and desired aesthetic qualities.
Anodizing Process
The anodizing process involves immersing the prepared steel workpieces in the electrolyte solution and applying a controlled electrical current. During this phase, oxygen ions are released from the electrolyte and react with the steel surface to form the anodized oxide layer. The duration of the anodizing process can vary based on the desired thickness of the oxide layer and the specific requirements of the application. Maintaining steady conditions throughout the process is essential for producing a high-quality anodized surface.
Time
The anodizing time directly affects the thickness and properties of the oxide layer. Longer anodizing times result in thicker oxide layers, which can provide greater corrosion resistance and surface hardness. However, excessive anodizing time can lead to increased production costs and potential over-thickening of the oxide layer, which may impact the mechanical properties and appearance of the steel parts. Balancing anodizing time with desired outcomes is critical for optimizing both performance and cost-efficiency.
Temperature
Temperature control during the anodizing process is essential to ensure consistent oxide layer formation. Elevated temperatures can accelerate the anodizing reaction, leading to faster oxide layer growth, while lower temperatures can slow the process and affect the uniformity of the oxide layer. Maintaining an optimal temperature range, typically between 20°C and 30°C, helps in achieving a stable and uniform anodized layer that meets the desired specifications.
Sealing
Sealing is the final step in the anodizing process, which involves hydrating the oxide layer to close its pores and enhance its protective qualities. This is typically achieved by immersing the anodized steel parts in boiling water or a sealing solution, allowing water molecules to bond with the oxide layer. Sealing improves corrosion resistance, reduces the likelihood of staining, and enhances the overall durability of the anodized surface. Proper sealing ensures that the anodized layer remains intact and effective in protecting the steel parts from environmental factors.
Anodizing methods vary based on the desired properties and applications of the anodized steel parts. Each method involves specific process parameters and electrolyte compositions to achieve unique characteristics in the oxide layer. Understanding the different anodizing techniques allows manufacturers to select the most appropriate method for their CNC machining services, ensuring that the anodized steel parts meet the required performance and aesthetic standards.
Sulfuric Acid Anodizing
Oxide Layer Thickness
Sulfuric acid anodizing is one of the most common methods used for anodizing steel. This process involves immersing the steel parts in a sulfuric acid electrolyte and applying an electrical current to facilitate oxide layer formation. Sulfuric acid anodizing typically produces oxide layers with thicknesses ranging from 10 to 50 microns, depending on the process parameters such as voltage, time, and temperature. The thickness of the oxide layer can be controlled by adjusting these parameters, allowing for customization based on the specific needs of the application.
Applications
Sulfuric acid anodizing is widely used in industries that require enhanced corrosion resistance and surface hardness for steel components. Applications include automotive parts, industrial machinery, construction elements, and consumer goods where durability and aesthetic appeal are important. The versatility and effectiveness of sulfuric acid anodizing make it a preferred choice for producing high-quality anodized steel CNC machining parts that can withstand harsh environments and heavy usage.
Hard Anodizing
Oxide Layer Thickness
Hard anodizing, also known as Type III anodizing, involves creating a much thicker and harder oxide layer compared to standard anodizing methods. This is achieved by using higher voltages and lower temperatures during the anodizing process, resulting in oxide layers with thicknesses ranging from 50 to 150 microns or more. The increased thickness significantly enhances the surface hardness and wear resistance of the steel parts, making them suitable for applications that demand exceptional durability.
Applications
Hard anodizing is ideal for applications that require superior wear resistance and longevity. Industries such as aerospace, automotive, heavy machinery, and tooling benefit from hard anodized steel parts due to their ability to withstand high stress, abrasion, and mechanical wear. Examples include gears, bearings, engine components, and industrial tools that require a hard, protective surface to maintain performance and reduce maintenance needs. The robust oxide layer provided by hard anodizing ensures that steel parts retain their functionality and appearance even under demanding conditions.

Chromic Acid Anodizing
Oxide Layer Thickness
Chromic acid anodizing utilizes a chromic acid electrolyte to form a thin, uniform oxide layer on steel surfaces. This method typically produces oxide layers with thicknesses between 5 to 20 microns. While thinner than those produced by sulfuric or hard anodizing, chromic acid anodizing provides excellent corrosion resistance and a smooth, glossy finish. The thin oxide layer also minimizes dimensional changes in the steel parts, maintaining tight tolerances and precise geometries essential for high-precision CNC machining applications.
Applications
Chromic acid anodizing is suitable for applications where a high-quality, corrosion-resistant finish is required without significantly altering the dimensions of the steel parts. Industries such as electronics, aerospace, and medical devices utilize chromic acid anodizing for components that demand both aesthetic appeal and reliable performance. Examples include connectors, housings, precision instruments, and other parts where maintaining dimensional accuracy and achieving a smooth surface finish are critical.
Oxalic Acid Anodizing
Oxide Layer Thickness
Oxalic acid anodizing involves using an oxalic acid electrolyte to produce a moderately thick oxide layer on steel surfaces. The resulting oxide layer typically ranges from 20 to 50 microns in thickness. This method provides a balance between corrosion resistance and surface hardness, making it suitable for applications that require durable yet lightweight steel parts. The oxide layer formed through oxalic acid anodizing offers good protection against environmental factors while maintaining the structural integrity and performance of the steel components.
Applications
Oxalic acid anodizing is utilized in applications where a durable and corrosion-resistant finish is needed without the extensive thickness of hard anodizing. Industries such as automotive, marine, and industrial manufacturing use oxalic acid anodizing for components like fasteners, fittings, and structural elements that require reliable protection against corrosion and wear. The versatility and moderate oxide layer thickness make oxalic acid anodizing a practical choice for producing steel CNC machining parts that need to balance durability with cost-effectiveness.
Phosphoric Acid Anodizing
Oxidizing
Phosphoric acid anodizing involves using a phosphoric acid electrolyte to create a protective oxide layer on steel surfaces. This method is particularly effective for enhancing corrosion resistance and surface hardness, providing a robust protective barrier that improves the durability and longevity of the steel parts. Phosphoric acid anodizing is known for its ability to form a uniform and adherent oxide layer that withstands harsh environmental conditions, making it ideal for demanding applications.
Thickness of Oxide Layer
The thickness of the oxide layer produced by phosphoric acid anodizing typically ranges from 30 to 80 microns, depending on the specific process parameters and requirements of the application. This moderate thickness provides a significant increase in surface hardness and corrosion resistance while maintaining the overall dimensional integrity of the steel parts. The controlled thickness ensures that the CNC machined parts retain their precise geometries and functional performance, making phosphoric acid anodizing a suitable choice for high-precision and high-performance applications.
Applications
Phosphoric acid anodizing is widely used in industries that require highly durable and corrosion-resistant steel components. Applications include automotive parts, industrial machinery, marine equipment, and infrastructure elements where exposure to corrosive environments is common. The enhanced surface properties provided by phosphoric acid anodizing make it ideal for parts that must maintain their integrity and functionality over extended periods, even under severe conditions. Examples include engine components, gears, fittings, and structural elements that benefit from the robust protection offered by phosphoric acid anodizing.
Anodizing steel offers numerous advantages that enhance the performance, durability, and aesthetic appeal of CNC machining parts. By applying this electrochemical surface treatment, manufacturers can significantly improve the corrosion resistance, surface hardness, and overall lifespan of steel components. Additionally, anodizing provides opportunities for customization and aesthetic enhancements, making it a valuable process for a wide range of applications. This section explores the key benefits of anodizing steel, highlighting why it is a preferred surface treatment method in various industries.

Corrosion Protection
One of the primary advantages of anodizing steel is the substantial improvement in corrosion resistance. The anodized oxide layer acts as a protective barrier that shields the underlying steel from environmental factors such as moisture, salt, chemicals, and pollutants. This enhanced protection is particularly beneficial for steel components used in outdoor, marine, and industrial environments where exposure to corrosive elements is common.
By preventing rust and oxidation, anodizing steel extends the lifespan of CNC machined parts, reducing the need for frequent maintenance and replacement. This not only lowers operational costs but also ensures the reliability and safety of the components in critical applications. For instance, in the automotive and aerospace industries, anodized steel parts maintain their structural integrity and performance over extended periods, even under harsh conditions.
Enhanced Wear Resistance
Anodizing significantly increases the surface hardness of steel, enhancing its wear resistance and reducing the likelihood of surface degradation. The anodized oxide layer is much harder than untreated steel, providing a robust shield against abrasion, scratching, and other forms of mechanical wear. This is particularly advantageous for CNC machining parts that are subjected to constant friction and movement, such as gears, bearings, and moving assemblies.
Improved wear resistance ensures that steel components retain their smooth surfaces and precise dimensions over time, maintaining their functionality and performance. This durability is essential in applications where precision and reliability are critical, such as in industrial machinery, automotive components, and medical devices. By minimizing wear and tear, anodizing extends the operational life of CNC machined parts, contributing to overall production efficiency and product quality.
Colored Appearance
Anodizing offers the unique advantage of providing a wide range of color options for steel parts through dyeing. This aesthetic enhancement not only improves the visual appeal of the components but also allows for color-coding and branding opportunities. Colored anodized steel parts are widely used in consumer electronics, architectural elements, automotive trim, and decorative applications where appearance is as important as functionality.
The ability to achieve vibrant and consistent colors through anodizing adds value to CNC machining parts, making them more attractive to end-users and differentiating products in the market. Additionally, the colored anodized layer can help in identifying different components or indicating specific functions within complex assemblies, enhancing usability and maintenance.
Improved Durability
Anodizing enhances the overall durability of steel parts by creating a stable and adherent oxide layer that is integral to the metal. This layer not only protects against corrosion and wear but also improves the resistance of the steel surface to thermal and chemical stress. As a result, anodized steel components are better equipped to withstand harsh operating conditions, extreme temperatures, and exposure to corrosive substances.
Improved durability ensures that CNC machined parts maintain their structural integrity and performance over time, reducing the risk of failures and downtime in critical applications. This reliability is essential in industries such as aerospace, automotive, construction, and medical devices, where the failure of a single component can have significant consequences. By enhancing the robustness of steel parts, anodizing contributes to the overall quality and dependability of manufactured products.
While anodizing steel offers numerous benefits, it also comes with certain limitations that manufacturers must consider. Understanding these drawbacks is essential for making informed decisions about whether anodizing is the right surface treatment method for specific CNC machining projects. This section outlines the primary limitations associated with anodizing steel, including the possibility of hydrogen embrittlement, dimensional changes, color restrictions, increased costs, and environmental issues.
Possibility of Hydrogen Embrittlement
Hydrogen embrittlement is a significant concern when anodizing steel, particularly high-strength alloys. During the anodizing process, hydrogen ions can diffuse into the steel, leading to the formation of brittle microstructures. This embrittlement reduces the ductility and toughness of the steel, making it more susceptible to cracking and failure under stress.
The risk of hydrogen embrittlement is higher in certain steel alloys, especially those with high carbon content or those that have undergone heat treatment. To mitigate this risk, manufacturers must carefully control the anodizing parameters, such as temperature and electrolyte composition, and implement proper post-anodizing treatments to remove hydrogen from the steel. Failure to address hydrogen embrittlement can compromise the mechanical properties and reliability of CNC machined parts, leading to premature failures and increased maintenance costs.
Dimensional Changes
Anodizing can cause dimensional changes in steel parts due to the formation of the oxide layer. As the oxide layer grows, it adds thickness to the surface, potentially altering the precise dimensions and tolerances of the CNC machined parts. This is particularly problematic for high-precision applications where tight tolerances are critical for proper fit and function.
Engineers must account for these dimensional changes during the design phase, adjusting the machining dimensions to compensate for the expected oxide layer thickness. Alternatively, dimensional measurements can be taken post-anodizing to ensure that parts meet the required specifications. However, these adjustments add complexity to the manufacturing process and may increase production time and costs.
Color Restrictions
While anodizing provides opportunities for coloring steel parts, there are limitations to the range and consistency of colors that can be achieved. The color options are often constrained by the anodizing method and the type of steel alloy being used. Achieving vibrant and uniform colors can be more challenging with steel compared to aluminum, limiting the aesthetic versatility of anodized steel parts.
Additionally, certain color dyes may not adhere as effectively to steel surfaces, resulting in uneven coloration or fading over time. Manufacturers seeking specific color requirements may find anodizing steel to be less flexible and reliable compared to other coloring methods, such as powder coating or paint application.
Increased Cost
Anodizing steel is generally more expensive than anodizing aluminum due to the additional complexities and requirements of the process. The higher material costs of steel, coupled with the need for specialized equipment and more stringent process controls, contribute to increased production expenses. Additionally, the potential for hydrogen embrittlement and the need for dimensional adjustments further escalate the costs associated with anodizing steel.
These higher costs can make anodizing steel less cost-effective for projects where the benefits do not outweigh the additional expenses. Manufacturers must carefully evaluate whether the enhanced properties provided by anodizing steel justify the increased production costs for their specific applications.
Environmental Issues
Anodizing processes, including those for steel, involve the use of hazardous chemicals and generate waste products that pose environmental concerns. Sulfuric acid and other electrolytes used in the anodizing process must be handled and disposed of properly to prevent environmental contamination. Additionally, the energy-intensive nature of anodizing can contribute to a larger carbon footprint, particularly when large-scale production is involved.
Manufacturers must implement stringent environmental management practices to comply with regulations and minimize the environmental impact of anodizing operations. This includes recycling and neutralizing waste acids, optimizing energy usage, and adopting eco-friendly anodizing techniques where possible. Failure to address environmental issues can result in regulatory penalties, reputational damage, and increased operational costs.
Anodizing steel, while beneficial, can present several potential problems that manufacturers need to be aware of. These issues can affect the quality, performance, and cost-effectiveness of the anodized CNC machining parts. Understanding these potential problems allows manufacturers to implement preventive measures and troubleshoot issues effectively, ensuring the success of their anodizing projects. This section outlines the primary challenges associated with anodizing steel, including the expense of the process, process complexity, and strict operating conditions.
Expensive Process
One of the most significant challenges of anodizing steel is the high cost associated with the process. Anodizing steel requires specialized equipment, high-quality electrolytes, and skilled technicians to manage the intricate anodizing parameters. The higher raw material costs of steel compared to aluminum also contribute to the overall expense. Additionally, the need for precise control over factors such as voltage, temperature, and electrolyte composition further increases production costs.
The expense of anodizing steel can make it less accessible for manufacturers with limited budgets or those producing low-volume CNC machining parts. For projects where the benefits of anodizing do not justify the additional costs, manufacturers may need to explore alternative surface treatment methods or reconsider the necessity of anodizing for certain components.
Process Complexity
Anodizing steel is inherently more complex than anodizing aluminum due to the material’s composition and the specific requirements of the anodizing process. Achieving a uniform and durable oxide layer on steel involves meticulous control over various process parameters, including voltage, temperature, and electrolyte composition. The presence of alloying elements in steel, such as chromium, nickel, and carbon, can affect the anodizing behavior and require additional adjustments to the process.
Furthermore, the risk of hydrogen embrittlement and the potential for dimensional changes add layers of complexity to the anodizing process. Manufacturers must have a deep understanding of the electrochemical principles involved and the ability to adjust the process in real-time to address any inconsistencies or defects in the oxide layer.
Strict Operating Conditions
The anodizing process for steel demands strict adherence to operating conditions to achieve the desired results. Deviations from optimal parameters can lead to defects in the oxide layer, such as uneven thickness, poor adhesion, or incomplete coverage. Maintaining consistent temperature, voltage, and electrolyte concentration is crucial for producing high-quality anodized steel parts.
Additionally, the need for specialized safety measures and environmental controls adds to the complexity of the anodizing operations. Manufacturers must implement robust quality control systems and continuously monitor process conditions to ensure that the anodizing process remains stable and produces reliable results.
Failure to maintain strict operating conditions can result in poor-quality anodized surfaces, increased defect rates, and higher production costs due to rework and scrap parts. Ensuring process stability and consistency is essential for maximizing the benefits of anodizing steel while minimizing potential problems.
While anodizing steel offers substantial benefits, it may not always be the most practical or cost-effective surface treatment option. Various alternatives can provide similar enhancements in corrosion resistance, surface hardness, and aesthetic appeal without the complexities and high costs associated with anodizing. Exploring these alternatives allows manufacturers to select the most appropriate surface treatment method based on their specific requirements, budget constraints, and application needs. This section examines several viable alternatives to anodized steel, including passivation, phosphating, and electrolytic polishing.
Passivation
Passivation is a chemical treatment process that enhances the corrosion resistance of stainless steel by removing free iron from the surface and promoting the formation of a passive oxide layer. Unlike anodizing, which thickens the existing oxide layer, passivation refines and stabilizes it, providing improved protection against environmental factors.
Process Overview:
Passivation typically involves immersing the stainless steel parts in an acid solution, such as nitric acid or citric acid, which reacts with the surface to remove contaminants and create a uniform oxide layer. This process does not significantly alter the dimensions or appearance of the steel parts, making it suitable for high-precision CNC machining applications.
Advantages:
Applications:
Passivation is widely used in industries such as automotive, medical devices, electronics, and food processing, where corrosion resistance is critical. Examples include surgical instruments, electronic housings, automotive components, and kitchen appliances.
Phosphating
Phosphating is a chemical conversion coating process that applies a layer of phosphate crystals to the surface of steel parts. This layer provides corrosion resistance, improves paint adhesion, and enhances the wear resistance of the components.
Process Overview:
Phosphating involves immersing the steel parts in a phosphoric acid solution, often containing zinc, manganese, or iron ions, which react with the surface to form a phosphate coating. The thickness and composition of the coating can be adjusted based on the specific requirements of the application.
Advantages:
Applications:
Phosphating is commonly used in the automotive industry for engine parts, chassis components, and body panels. It is also utilized in industrial machinery, hardware, and tools where improved paint adhesion and corrosion resistance are essential.
Electrolytic Polishing
Electrolytic polishing, also known as electropolishing, is an electrochemical process that removes a thin layer of material from the surface of steel parts, resulting in a smooth and shiny finish. This process enhances the surface quality and corrosion resistance of the components.
Process Overview:
Electropolishing involves immersing the steel parts in an electrolyte solution and applying an electric current. The anodic reaction removes microscopic surface imperfections, resulting in a highly polished and passivated surface.
Advantages:
Applications:
Electrolytic polishing is widely used in the medical industry for surgical instruments and implants, in the food and beverage industry for processing equipment, and in the electronics industry for precision components.

Anodized steel finds its application across a diverse range of industries due to its enhanced corrosion resistance, surface hardness, and aesthetic appeal. The anodized oxide layer provides both functional and aesthetic benefits, making anodized steel a valuable material for various high-performance and decorative applications. This section explores the specific industries and applications where anodized steel is commonly utilized, highlighting the advantages it offers in each context.
Architectural
In the architectural sector, anodized steel is prized for its ability to withstand harsh environmental conditions while maintaining an attractive appearance. Anodized steel is used in building facades, window frames, door hardware, and decorative panels. The corrosion-resistant properties ensure that architectural elements remain durable and visually appealing over time, even in outdoor settings exposed to moisture, pollution, and varying temperatures. Additionally, the ability to achieve a range of colors through anodizing allows architects to incorporate vibrant and consistent finishes that complement the overall design aesthetic.
The automotive industry leverages anodized steel for various components that require both strength and corrosion resistance. Applications include engine parts, suspension components, trim pieces, and decorative elements. Anodized steel parts in vehicles benefit from enhanced durability and reduced maintenance needs, as the anodized layer protects against rust and wear caused by road conditions and exposure to automotive fluids. Furthermore, the aesthetic versatility of anodized steel allows manufacturers to achieve specific color finishes that align with vehicle branding and design requirements.
Aerospace
In the aerospace sector, anodized steel is used for critical components that demand high performance, reliability, and resistance to extreme conditions. Applications include structural elements, fasteners, and control systems. The anodized oxide layer enhances the corrosion resistance and surface hardness of steel parts, ensuring that they can withstand the rigorous demands of aerospace environments, such as high altitudes, temperature fluctuations, and exposure to corrosive agents. Anodized steel contributes to the overall safety and longevity of aerospace components, making it an essential material in the industry.
Consumer Goods
Anodized steel is increasingly popular in the consumer goods industry for products that require a combination of durability and aesthetic appeal. Applications include kitchen appliances, furniture, electronics casings, and sporting goods. The anodized finish not only enhances the visual appeal of these products but also provides a protective barrier against everyday wear and tear, ensuring that consumer goods remain functional and attractive over extended use. Additionally, the ability to achieve various colors and finishes through anodizing allows manufacturers to offer a wide range of product designs that cater to diverse consumer preferences.
In the medical device industry, anodized steel is used for surgical instruments, implants, and other medical equipment that require high levels of cleanliness, corrosion resistance, and biocompatibility. The anodized oxide layer provides a smooth and hygienic surface that is resistant to bacterial growth and easy to sterilize. Additionally, the enhanced surface hardness contributes to the durability and longevity of medical instruments, ensuring that they maintain their precision and functionality over multiple uses. Anodized steel is thus a critical material in the production of reliable and safe medical devices.
Anodized steel is utilized in the electronics industry for components that require high precision, durability, and aesthetic quality. Applications include connectors, housings, frames, and other structural elements of electronic devices. The anodized oxide layer provides excellent corrosion resistance, ensuring that electronic components remain reliable and functional over time. Furthermore, the ability to achieve sleek and uniform finishes through anodizing enhances the overall appearance of electronic products, making them more appealing to consumers.
Before carrying out the anodizing process, ensuring that the metal surface meets the appropriate preconditions is essential. These preconditions not only determine the effectiveness and final quality of anodizing but also directly affect the performance and appearance of the product. Below are the key preconditions for the anodizing process:
1. Surface Cleanliness
Surface cleanliness is a crucial step in the anodizing process, aimed at removing oils, fats, oxides, and other contaminants from the metal surface. An unclean surface can lead to uneven formation of the oxide layer, which can impact both its protective properties and visual quality. Common cleaning methods include:
2. Surface Roughness (Optional)
Depending on the specific application, it may be necessary to roughen the metal surface before anodizing to enhance the adhesion and mechanical properties of the oxide layer. Surface roughening can be achieved through mechanical methods (such as sandblasting or grinding) or chemical methods. A rough surface increases the surface area for the oxide layer, improving its wear resistance and corrosion protection. Surface roughening is particularly important for high-precision applications, such as automotive components and industrial machinery.
3. Surface Polishing (Optional)
Surface polishing is an additional step to improve the quality of the metal surface, especially for anodizing applications that require a high degree of surface smoothness and fine texture. The polishing process eliminates minor surface defects and inconsistencies, resulting in a smoother and more uniform surface roughness. This not only ensures an even oxide layer but also enhances the final product's appearance and functionality. This treatment is widely used in the anodizing of precision electronic components and consumer products.
4. Pretreatment Steps
After surface cleaning, roughening, and polishing, it is typically necessary to perform a conditioning step to further prepare the metal surface for the anodizing process. This includes neutralizing any residual acids or alkalines, ensuring the surface pH is within the proper range, and performing a final rinse to remove any chemical residues. The goal of this pretreatment is to provide stable and consistent starting conditions for the anodizing process, ensuring the quality and performance of the oxide layer.
5. Process Parameters
The success of the anodizing process also depends on strict control of process parameters, including electrolyte concentration, temperature, voltage, and processing time. Different metals and alloys require different parameters, so these must be adjusted and optimized according to the specific material. By precisely controlling the process parameters, the desired thickness and properties of the oxide layer can be achieved, meeting the requirements for various applications.
6. Quality Control and Inspection
Quality control before anodizing is also crucial to ensure the effectiveness of the process. Surface roughness tests, chemical composition analysis, and microscopic observation can assess the readiness of the metal surface, ensuring it meets the requirements for anodizing. Proper quality pre-treatment not only enhances the anodizing effect but also reduces defects and rework in subsequent processes, improving overall production efficiency and product quality.
Conclusion
The preconditions for anodizing include thorough surface cleaning, appropriate surface roughening and precise treatment, accurate control of process parameters, and strict quality inspection. These preconditions work together to ensure the smooth operation of the anodizing process, resulting in a high-quality oxide layer that enhances the corrosion resistance, surface hardness, and aesthetics of metal components. For CNC machining factories, prioritizing and optimizing these preconditions is key to producing high-performance, durable, and aesthetically pleasing custom CNC machined parts.
Understanding the differences between anodizing steel and aluminum is essential for manufacturers to select the appropriate material and process for their CNC machining projects. While both metals can be anodized to enhance their surface properties, the processes, resulting oxide layers, and applications differ significantly.
Anodizing Aluminum:
Anodizing Steel:
Key Differences:
1. Material Behavior: Aluminum forms a stable and protective oxide layer more easily, making anodizing simpler and more reliable. Steel, especially certain alloys, requires more controlled conditions to form a consistent oxide layer.
2. Process Requirements: Anodizing steel often necessitates higher voltages, specialized electrolytes, and more rigorous surface preparation compared to aluminum.
3. Cost and Complexity: The anodizing process for steel is generally more expensive and complex due to the additional steps and precision required to achieve high-quality results.
4. Application Suitability: Anodized aluminum is preferred for applications where weight reduction and aesthetic versatility are paramount, while anodized steel is chosen for its superior strength and durability in demanding environments.
By recognizing these differences, manufacturers can make informed decisions about whether to anodize steel or aluminum based on their specific CNC machining needs, budget constraints, and desired outcomes.
At VMT Precision Machining Services, we specialize in providing high-quality anodizing services for steel CNC machined parts, enhancing their performance, durability, and aesthetic appeal. Our state-of-the-art CNC machining factory is equipped with advanced anodizing equipment and staffed by experienced technicians who understand the complexities of anodizing steel. Whether you require standard anodizing for improved corrosion resistance or hard anodizing for enhanced wear resistance, VMT Precision Machining Services delivers tailored solutions that meet your specific needs.

Our Anodizing Services Include:
By partnering with VMT Precision Machining Services for your steel anodizing needs, you benefit from our expertise in both CNC machining and surface treatments. We work closely with you to understand your project goals and deliver anodized steel CNC machining parts that exceed your expectations in terms of performance, appearance, and longevity. Whether you need small-scale prototype machining or high-volume production runs, our CNC machining services are designed to provide efficient, cost-effective, and high-quality solutions tailored to your unique requirements.
Anodizing steel represents a significant advancement in CNC machining parts manufacturing, offering enhanced corrosion resistance, surface hardness, and aesthetic versatility. While the anodizing process for steel is more complex and costly compared to aluminum, the benefits it provides in terms of durability, performance, and appearance make it a valuable surface treatment option for a wide range of applications. By understanding the process, advantages, and potential limitations of steel anodizing, manufacturers can make informed decisions that optimize their production processes and deliver superior CNC machined parts.
Furthermore, exploring alternatives such as passivation, phosphating, and electrolytic polishing offers additional avenues for enhancing the surface properties of steel components without the high costs and complexities associated with anodizing. Each alternative presents its own set of benefits and is suited to different application requirements, allowing manufacturers to select the most appropriate surface treatment method based on their specific needs and constraints.
Partnering with experienced CNC machining services like VMT Precision Machining Services ensures that your anodized steel CNC machining parts are produced with the highest standards of quality and precision. Our expertise in both machining and surface treatments enables us to deliver customized solutions that meet the demanding requirements of various industries, from automotive and aerospace to medical devices and consumer electronics.
By embracing the capabilities of anodizing and its alternatives, CNC machining factories can enhance their product offerings, reduce maintenance costs, and extend the lifespan of their components, thereby driving innovation and maintaining a competitive edge in the dynamic manufacturing landscape.
What are the Best Metals for Anodizing?
The best metals for anodizing are aluminum and titanium due to their excellent ability to form stable and protective oxide layers. These metals respond well to the anodizing process, resulting in enhanced corrosion resistance, surface hardness, and aesthetic finishes. Other metals such as magnesium and certain types of steel can also be anodized, but they require more specialized processes and control to achieve similar results.
Can Mild Steel Be Anodized?
Yes, mild steel can be anodized, but the process is more challenging compared to anodizing aluminum. Mild steel requires precise control over the anodizing conditions, including voltage, temperature, and electrolyte composition, to form a consistent and protective oxide layer. The resulting anodized layer on mild steel enhances its corrosion resistance and surface hardness, making it suitable for applications that demand durability and protection. However, achieving uniform anodizing on mild steel often necessitates additional surface preparation and specialized equipment, increasing the complexity and cost of the process.
Can Stainless Steel Be Anodized?
Yes, stainless steel can be anodized, particularly high-chromium stainless steel alloys. The anodizing process for stainless steel involves creating a stable and uniform oxide layer that enhances corrosion resistance and surface hardness. This treatment is beneficial for stainless steel parts used in harsh environments, as it provides additional protection against rust and wear. However, anodizing stainless steel is more complex and costly compared to anodizing aluminum, requiring specialized processes and precise control over anodizing parameters to achieve consistent results.
How to Tell if a Metal is Anodized?
To determine if a metal has been anodized, you can look for certain visual and physical indicators:
1. Color Consistency: Anodized metals often have a uniform and vibrant color finish that is consistent across the entire surface.
2. Texture: Anodized surfaces typically feel smoother and harder compared to untreated metal surfaces.
3. Resistance to Corrosion: Anodized metals exhibit enhanced corrosion resistance, making them less prone to rust and degradation in harsh environments.
4. Color Stability: The color of anodized metals remains stable over time and is resistant to fading from exposure to sunlight and chemicals.
5. Inspection Tests: Conducting specific tests, such as using a scratch test to check the adherence of the oxide layer or performing a corrosion resistance test, can confirm anodizing.
Additionally, manufacturers can provide certification or documentation indicating that the metal has undergone anodizing treatment.
Can Titanium Be Anodized?
Yes, titanium is highly receptive to anodizing and is one of the most popular metals to anodize after aluminum. The anodizing process for titanium involves creating a controlled oxide layer that enhances its corrosion resistance, surface hardness, and aesthetic appeal. Titanium anodizing is widely used in applications requiring biocompatibility, such as medical implants, as well as in aerospace, automotive, and consumer electronics for its lightweight and durable properties. The ability to achieve a variety of colors through anodizing also makes titanium an attractive choice for decorative and branding purposes.
Which is Better to Anodize Stainless Steel or Aluminum?
Aluminum is generally easier and more cost-effective to anodize compared to stainless steel. Aluminum forms a stable and protective oxide layer naturally, making the anodizing process simpler and more reliable. The oxide layer on anodized aluminum provides excellent corrosion resistance, surface hardness, and aesthetic versatility through color dyeing.
Stainless steel, while anodizable, requires more specialized processes and precise control to achieve a uniform and effective oxide layer. Anodizing stainless steel is more complex and costly, but it offers enhanced corrosion resistance and surface hardness for applications that demand higher durability.
In summary, anodized aluminum is typically the better choice for applications where cost, ease of anodizing, and aesthetic flexibility are prioritized, while anodized stainless steel is preferred for applications that require superior strength and corrosion resistance despite the higher costs and complexity involved.
Which Metals Cannot Be Anodized?
While many metals can undergo anodizing, some are less suitable or cannot be anodized effectively. Metals that do not form a stable and protective oxide layer or have incompatible electrochemical properties are challenging to anodize. Examples include:
These metals may require alternative surface treatment methods to achieve desired protective or aesthetic qualities.
What is the Purpose of Anodizing?
The primary purpose of anodizing is to enhance the surface properties of metals by creating a protective oxide layer. This process improves corrosion resistance, surface hardness, and wear resistance, while also providing opportunities for aesthetic enhancements such as coloring and surface texture modification. Anodizing serves both functional and decorative purposes, making metals more durable and visually appealing for a wide range of applications.
In CNC machining parts manufacturing, anodizing ensures that metal components are protected from environmental factors, extend their lifespan, and achieve the desired surface finishes that meet specific design and performance requirements.
Which is Better to Anodize, Hard Anodizing or Stainless Steel?
The decision to anodize hard anodized aluminum or anodized stainless steel depends on the specific application requirements:
In summary, hard anodizing aluminum is better for applications where lightweight and high surface hardness are prioritized, while anodized stainless steel is better for applications requiring maximum strength and durability despite the higher costs and complexity.
Can Anodized Aluminum Replace Stainless Steel in Industrial Applications?
Anodized aluminum can replace stainless steel in certain industrial applications where the benefits of aluminum—such as lightweight, excellent corrosion resistance, and aesthetic versatility—are more advantageous than the superior strength and durability of stainless steel. However, this replacement is dependent on the specific requirements of the application.
Advantages of Replacing Stainless Steel with Anodized Aluminum:
Limitations:
Manufacturers must evaluate the specific demands of their industrial applications to determine if anodized aluminum can effectively replace stainless steel without compromising performance and reliability.
How Does the Durability of Anodized Aluminum Compare to Anodized Steel?
Anodized aluminum and anodized steel both benefit from enhanced durability due to their protective oxide layers, but they offer different advantages based on their inherent material properties:
Anodized Aluminum:
Anodized Steel:
In conclusion, anodized aluminum is more durable in terms of corrosion resistance and surface hardness while being lightweight, whereas anodized steel excels in strength, wear resistance, and durability under high-stress conditions. The choice between the two depends on the specific durability requirements of the application.
What Industries Commonly Use Anodized Aluminum?
Anodized aluminum is widely utilized across numerous industries due to its enhanced surface properties and aesthetic appeal. Some of the key industries that commonly use anodized aluminum include:
1. Automotive: For engine components, trim pieces, and decorative elements that require lightweight, corrosion-resistant finishes.
2. Aerospace: In aircraft structures, interior components, and heat exchangers where durability and lightweight properties are essential.
3. Consumer Electronics: For device housings, heat sinks, and internal components that benefit from enhanced corrosion resistance and aesthetic versatility.
4. Architecture: In building facades, window frames, door hardware, and decorative panels that require durable and attractive finishes.
5. Medical Devices: For surgical instruments, implants, and equipment that demand high levels of cleanliness, corrosion resistance, and biocompatibility.
6. Sporting Goods: In bike frames, helmets, and other equipment where lightweight and durable finishes are crucial.
7. Furniture: For decorative and structural elements that require a polished and corrosion-resistant surface.
8. Industrial Machinery: In parts that require enhanced wear resistance and corrosion protection for prolonged use in harsh environments.
The versatility and reliability of anodized aluminum make it a preferred choice for applications that demand both functional performance and aesthetic quality.
This comprehensive article addresses the process, advantages, and alternatives to anodizing steel, providing valuable insights for manufacturers in the CNC machining parts industry. By understanding the complexities and benefits of steel anodizing, alongside viable alternatives, manufacturers can make informed decisions that enhance the quality, durability, and aesthetic appeal of their CNC machined parts.
