15 years one-stop China custom CNC machining parts factory
 323 |
Published by VMT at Sep 02 2025 | Reading Time:About 7 minutes
323 |
Published by VMT at Sep 02 2025 | Reading Time:About 7 minutes
If you’ve ever handled “rusty” components or specified ferric materials in CNC machined parts, you’ve probably wondered: is iron oxide (ferric oxide) magnetic or not—and will it interfere with tolerances, sensors, or assembly? The problem is that “iron oxide (ferric oxide)” isn’t one thing; it’s a family ranging from strongly magnetic magnetite to weak or practically non-magnetic forms of rust. Confusion here leads to inspection headaches, stray chip attraction on machines, and even quality escapes. The good news: once you understand which oxides are magnetic, why they behave differently, and how processing affects them, you can design and source confidently. In this step-by-step guide from a VMT CNC machining perspective, we’ll demystify “iron oxide (ferric oxide) magnetic” behavior, compare it to other materials.
Some iron oxides (ferric oxides) are magnetic. Magnetite (Fe₃O₄) is strongly magnetic; maghemite (γ-Fe₂O₃) is ferrimagnetic but weaker; common rust (α-Fe₂O₃ hematite) is weakly magnetic at room temperature. Knowing which oxide you have prevents contamination, measurement errors, and rework in CNC machining services and CNC machining factories.
You’ll see phrases like “iron oxide (ferric oxide) magnetic,” “ferric oxide magnetic,” and even “non-magnetic iron oxide (ferric oxide)” used interchangeably online, which fuels confusion in engineering notes and supplier quotes. In reality, iron oxides (ferric oxides) differ in crystal structure, defect chemistry, particle size, and processing history, and these factors determine whether they behave like a ferromagnet/ferrimagnet (strong attraction), a paramagnet (weak, field-dependent), or appear practically non-magnetic in shop conditions. That distinction matters on the shop floor: magnetic debris sticks to cutters, probes may misread, and parts can attract fines that compromise coatings or assemblies. As we walk through definitions, types, and the physics behind magnetism, we’ll tie every concept back to purchasing, inspection, and process control for CNC machined parts. When you’re ready for more hands-on guidance, check our [CNC Machined Parts Materials Library] and [Cleaning & Passivation Best Practices] to see how material choice and finishing eliminate magnetism-related risks.
Yes, some iron oxides (ferric oxides) are magnetic, but some are not. it depends on the type of iron oxide (ferric oxide). Not all iron oxides (ferric oxides) exhibit the same level of magnetism. Magnetite (Fe₃O₄) is strongly magnetic and is classified as a ferrimagnetic material. This makes it one of the most important naturally occurring magnetic minerals, widely used in electronics, recording media, and industrial applications. Maghemite (γ-Fe₂O₃), sometimes referred to as magnetic hematite, is also ferrimagnetic but weaker than magnetite. On the other hand, common rust, which is usually hematite (α-Fe₂O₃), is either weakly magnetic or practically non-magnetic at room temperature. This variation explains why you may notice some iron oxides (ferric oxides) clinging to a magnet while others show no response.
In CNC machining services, understanding whether iron oxide (ferric oxide) is magnetic is more than just a scientific curiosity. Residual magnetic oxides on CNC machined parts can attract metallic fines during production, interfere with inspection probes, and affect assembly performance. CNC machining factories often implement cleaning, passivation, or coating processes to ensure that unwanted magnetic behavior does not compromise part quality. When engineers specify “ferric oxide magnetic properties” without detail, suppliers may misinterpret, leading to mismatched expectations and higher project costs.

Iron oxide is a general term for compounds formed when iron reacts with oxygen. Chemically, these oxides are combinations of iron atoms (Fe) and oxygen atoms (O) in different ratios and crystal structures. The most common forms include magnetite (Fe₃O₄), hematite (Fe₂O₃), and wüstite (FeO), each with distinct physical and magnetic properties. Collectively, they appear in nature as minerals, in industrial pigments, or as corrosion layers—better known as rust—on iron and steel.
From a scientific perspective, the different valence states of iron (Fe²⁺ and Fe³⁺) explain why iron oxides (ferric oxides) vary so much in color, stability, and magnetism. For instance, magnetite contains both Fe²⁺ and Fe³⁺, giving it strong ferrimagnetic properties, while hematite, composed only of Fe³⁺, is weakly magnetic or nearly non-magnetic under everyday conditions. These variations are critical not only in geology and materials science but also in industries that rely on precise material selection.
In CNC machining factories, iron oxide (ferric oxide) usually appears as surface oxidation or rust on CNC machined parts. If uncontrolled, this oxide layer can impair dimensional accuracy, disrupt surface finishes, and introduce contamination during assembly. While some oxides such as magnetite are exploited for functional coatings, others like hematite represent unwanted corrosion, raising costs in rework or protective finishing. Understanding whether an oxide is magnetic or not helps machinists and engineers choose appropriate CNC machining services, cleaning processes, and coatings to extend part life.

Iron oxide is not a single material but a family of compounds with unique chemical and magnetic properties. The three most discussed varieties are magnetite (Fe₃O₄), maghemite (γ-Fe₂O₃, often called magnetic hematite), and non-magnetic oxides such as α-Fe₂O₃ (hematite). Each has a different crystal structure, electron configuration, and response to magnetic fields, which is why some are strongly magnetic while others barely interact with a magnet. For industries such as CNC machining services, knowing the difference is essential. Magnetic oxides can cause chips or dust to cling to CNC machined parts, while non-magnetic oxides are more often associated with corrosion or rust control.
Magnetite (Fe₃O₄)
Magnetite is the most magnetic of all naturally occurring iron oxides (ferric oxides). Its crystal structure contains both Fe²⁺ and Fe³⁺ ions, which create a ferrimagnetic alignment of spins, resulting in strong magnetism. This makes Fe₃O₄ widely used in magnetic recording media, catalysts, ferrofluids, and industrial coatings. In machining environments, magnetite layers sometimes form intentionally during controlled oxidation processes to provide a degree of wear and corrosion resistance. However, uncontrolled magnetite buildup on CNC machined parts can interfere with dimensional precision and surface finishes.
For CNC machining factories, magnetite contamination poses the risk of attracting metallic swarf, which can damage cutting edges or embed into part surfaces. Controlled cleaning or protective coatings are required to maintain high-quality finishes.
Magnetic Hematite (γ-Fe₂O₃)
Maghemite (γ-Fe₂O₃), often called magnetic hematite, is another iron oxide (ferric oxide) that exhibits ferrimagnetism, though weaker than magnetite. It is chemically similar to hematite but has a defect-rich spinel structure that allows magnetic domains to form. Maghemite is widely used in pigments, magnetic tapes, and biomedical imaging. In practice, its magnetism is sufficient to attract fine metallic particles, but it is not as strong as Fe₃O₄.
In CNC machining, maghemite layers may form during high-temperature oxidation of iron or steel. While less problematic than magnetite, these magnetic oxides still demand attention, especially in precision CNC machining services where debris attraction can compromise inspection accuracy. For CNC machining factories, ensuring the oxide state is well understood helps minimize rework and prevent part rejection.
Non-Magnetic Iron Oxide (α-Fe₂O₃ and others)
The most common form of non-magnetic iron oxide (ferric oxide) is hematite (α-Fe₂O₃), better known as rust. At room temperature, hematite is weakly magnetic or effectively non-magnetic, which explains why most rusted iron surfaces don’t stick to magnets. Other forms, such as wüstite (FeO), also show minimal magnetic response under typical conditions. These oxides generally result from uncontrolled corrosion and are rarely desirable on precision components.
For CNC machined parts, non-magnetic oxides are a red flag, indicating corrosion and potential dimensional instability. Unlike magnetite, these oxides provide little protection and often flake off, exposing fresh metal to further oxidation. This increases maintenance costs and complicates storage and shipping in CNC machining factories.
Types of Iron Oxide (Ferric Oxide)
| Type of Iron Oxide (Ferric Oxide) |
Chemical Formula |
Magnetism |
Crystal Structure |
Common Occurrence |
Impact on CNC Machined Parts |
| Magnetite |
Fe₃O₄ | Strongly magnetic (ferrimagnetic) | Inverse spinel (mixed Fe²⁺/Fe³⁺) | Natural mineral, controlled oxidation coatings | Attracts chips/dust, may disrupt tolerances; can be used as protective layer if intentional |
| Maghemite (Magnetic Hematite) |
γ-Fe₂O₃ | Moderately magnetic (ferrimagnetic, weaker than Fe₃O₄) | Defect spinel (all Fe³⁺, oxygen vacancies) | High-temp oxidation products, pigments, magnetic media | Can attract fine debris; requires cleaning in precision CNC machining services |
| Hematite (Rust) |
α-Fe₂O₃ | Weakly magnetic / nearly non-magnetic | Corundum structure (Fe³⁺ only) | Common rust, corrosion on steel | Indicates corrosion, reduces part reliability; non-protective and prone to flaking |
| Wüstite |
FeO | Very weakly magnetic / paramagnetic | Rock salt structure (Fe²⁺ only) | High-temp reduction environments | Rare in CNC machining parts; unstable in air, usually converts to other oxides |
The magnetism of iron oxide (ferric oxide) comes down to physics at the atomic level. While all iron oxides (ferric oxides) are formed from iron and oxygen, not all share the same electronic arrangement. Magnetite (Fe₃O₄) and maghemite (γ-Fe₂O₃) are magnetic because their internal structures allow unpaired electrons to align into stable magnetic domains. In contrast, hematite (α-Fe₂O₃) is weakly magnetic at room temperature because its electron spins largely cancel out. For engineers working with CNC machined parts, this difference is more than theoretical—magnetic oxides can attract machining chips and interfere with sensitive measurement tools, while non-magnetic oxides often signal corrosion.
Atomic Structure
At the atomic level, magnetism originates from the behavior of electrons. In magnetite, both Fe²⁺ and Fe³⁺ ions are present in a crystal lattice. Their unpaired d-orbital electrons create spin moments that do not completely cancel out, resulting in ferrimagnetism—a strong form of magnetism observed even at room temperature. Maghemite also contains Fe³⁺ ions, but due to oxygen vacancies in its lattice, it still supports ferrimagnetic ordering, though weaker. Hematite, on the other hand, consists solely of Fe³⁺ ions in a structure where spins mostly cancel, making it only weakly magnetic.
For CNC machining services, this explains why different oxides form during heat treatment or surface exposure. Controlled magnetite layers may be beneficial, while unwanted hematite rust indicates poor protection.
Magnetic Domains
Beyond atomic structure, magnetism depends on magnetic domains—tiny regions where groups of atoms align their magnetic moments in the same direction. In strongly magnetic oxides like magnetite, domains naturally align, giving the material a net magnetic field. In non-magnetic oxides like hematite, domains are either absent or cancel each other, producing no external magnetism.
In CNC machining factories, even thin magnetic domains can cause challenges. For instance, if parts unintentionally acquire magnetized oxide patches, they may attract ferrous dust, complicating cleaning or causing premature tool wear. On the other hand, intentional magnetization can be useful in applications like magnetic sensors or specialized CNC machined parts for electronics.
Whether iron oxide (ferric oxide) attracts magnets depends entirely on the oxide type and its crystal structure. Magnetite (Fe₃O₄) is strongly magnetic and readily attracted to a magnet because it is a ferrimagnetic material. Maghemite (γ-Fe₂O₃) also attracts magnets but less strongly, as its ferrimagnetic ordering is weaker. In contrast, common rust (hematite, α-Fe₂O₃) is only weakly magnetic at room temperature, meaning a regular magnet will show little to no attraction. Similarly, wüstite (FeO) is practically non-magnetic under everyday conditions.
This explains why people are sometimes confused when they try to pick up rust flakes with a magnet—sometimes they stick, sometimes they don’t. The variation depends on which oxide phase formed during corrosion. For industries such as electronics, coatings, and especially CNC machining services, this distinction is critical. Magnetic oxides can attract machining debris and iron filings, which may contaminate sensitive CNC machined parts, disrupt finishing processes, or compromise dimensional inspection. Non-magnetic oxides, on the other hand, are usually a sign of uncontrolled corrosion rather than magnetic interference.
From a practical standpoint in CNC machining factories, engineers and machinists must consider whether residual oxides will interfere with product performance. For example, in aerospace and medical applications, even slight magnetic attraction can cause problems with sensors or precision assemblies. That’s why oxide control—through coatings, passivation, or surface treatments—is built into machining workflows.
The properties of iron oxide (ferric oxide) vary widely depending on its specific form—magnetite (Fe₃O₄), maghemite (γ-Fe₂O₃), hematite (α-Fe₂O₃), or wüstite (FeO). These oxides differ in chemical composition, color, hardness, and magnetic behavior. For engineers and designers sourcing CNC machined parts, understanding these variations is vital because oxide layers affect not only corrosion resistance but also the machining, cleaning, and assembly processes in CNC machining factories. A well-defined property profile helps ensure the chosen oxide supports, rather than hinders, functional requirements.
Chemical Composition of Iron Oxide (Ferric Oxide)
Iron oxides are composed of iron (Fe) and oxygen (O) in different ratios and oxidation states.
This variation in iron valence states explains why some oxides are strongly magnetic while others are non-magnetic. In CNC machining services, controlling the oxidation state is important, since unwanted Fe²⁺/Fe³⁺ imbalances can alter surface reactivity, leading to corrosion or magnetic contamination of machined components.
Chemical Composition of Iron Oxide (Ferric Oxide)
| Type of Iron Oxide (Ferric Oxide) |
Formula |
Iron Oxidation States |
Fe:O Ratio |
Key Notes |
| Magnetite |
Fe₃O₄ | Fe²⁺ + Fe³⁺ (mixed valence) | 3:4 | Strongly magnetic; stable black mineral; useful in coatings and electronics |
| Maghemite (Magnetic Hematite) |
γ-Fe₂O₃ | Fe³⁺ only (with oxygen vacancies) | 2:3 | Moderately magnetic; defect spinel structure; common in pigments & recording media |
| Hematite (Rust) |
α-Fe₂O₃ | Fe³⁺ only | 2:3 | Weakly magnetic at room temp; most stable oxide; forms as rust on steel |
| Wüstite |
FeO | Fe²⁺ only | 1:1 | Very weakly magnetic; unstable in air; forms in high-temp reduction conditions |
Physical Properties of Iron Oxide (Ferric Oxide)
The physical properties of iron oxides (ferric oxides) are just as diverse:
In CNC machining factories, these physical properties matter in practice. A hard, stable oxide like hematite can interfere with coating adhesion and accelerate tool wear if not removed. Magnetic oxides like magnetite can attract machining debris, complicating cleaning and inspection. Correctly identifying and managing oxide properties ensures consistent quality in CNC machined parts.
Physical Properties of Iron Oxide (Ferric Oxide)
| Type of Iron Oxide (Ferric Oxide) |
Color |
Density (g/cm³) |
Hardness (Mohs) |
Magnetism |
Stability |
| Magnetite (Fe₃O₄) |
Black / dark gray | ~5.2 | ~5.5 | Strongly magnetic (ferrimagnetic) | Stable under normal conditions; used in coatings & electronics |
| Maghemite (γ-Fe₂O₃) |
Brownish-red | ~4.9–5.0 | ~5.0 | Moderately magnetic (ferrimagnetic, weaker than Fe₃O₄) | Stable but can transform to hematite over time |
| Hematite (α-Fe₂O₃) |
Red to reddish-brown | ~5.3 | 5.5–6.5 | Weakly magnetic / nearly non-magnetic | Most thermodynamically stable iron oxide (ferric oxide) (common rust) |
| Wüstite (FeO) |
Black | ~5.7 | ~4.5 | Very weak (paramagnetic) | Unstable in air; oxidizes to other iron oxides (ferric oxides) |
The magnetic behavior of iron oxide (ferric oxide) is not constant; it depends on several external and internal factors. Variations in temperature, purity, crystal structure, external magnetic fields, and alloying elements can either enhance or diminish its magnetism. Understanding these influences is essential in fields like electronics, materials science, and CNC machining, where iron oxide (ferric oxide) coatings or components are used.
Temperature
Purity
Crystal Structure
External Magnetic Field
Alloying Elements
Here’s a clear comparison table for the factors affecting the magnetism of iron oxide (ferric oxide):
| Factor |
Effect on Magnetism |
| Temperature |
Above the Curie temperature (e.g., ~585 °C for Fe₃O₄), iron oxide (ferric oxide) loses permanent magnetism. |
| Purity |
High-purity iron oxide (ferric oxide) shows stronger magnetism; impurities (Si, Al, S) reduce domain alignment. |
| Crystal Structure |
Spinel forms (Fe₃O₄, γ-Fe₂O₃) are ferrimagnetic; hematite (α-Fe₂O₃) is weakly magnetic or non-magnetic. |
| External Magnetic Field |
Aligns magnetic domains, increasing magnetism; some oxides retain magnetism after field removal (permanent), others lose it. |
| Alloying Elements |
Magnetic dopants (Co, Mn, Ni) enhance magnetism; non-magnetic dopants (Ti, Al) weaken it. |
Iron oxide plays a central role in the study of magnetism because some of its forms display ferromagnetism or ferrimagnetism, while others do not. Ferromagnetism is the property of certain materials to exhibit strong, permanent magnetism due to the parallel alignment of electron spins within their atomic structure.
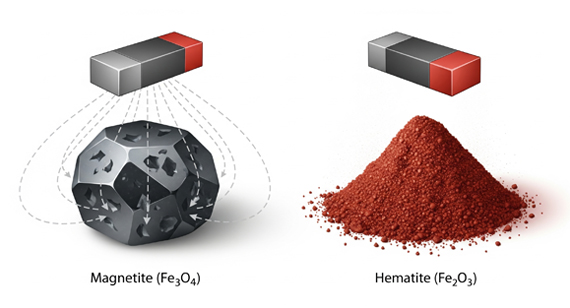
1. Magnetite (Fe₃O₄) – Strongly Ferrimagnetic
2. Maghemite (γ-Fe₂O₃) – Ferrimagnetic
3. Hematite (α-Fe₂O₃) – Weakly Magnetic (Antiferromagnetic)
4. Key Point: Not All Iron Oxides (Ferric Oxides) Are Ferromagnetic
Here’s a clear comparison table on Iron Oxide (Ferric Oxide) and Ferromagnetism:
| Type of Iron Oxide (Ferric Oxide) |
Chemical Formula |
Magnetic Behavior |
Mechanism |
Notes / Applications |
| Magnetite |
Fe₃O₄ | Ferrimagnetic (strongly magnetic) | Unequal but opposite magnetic moments of Fe²⁺ and Fe³⁺ ions → net magnetization | Used in magnetic storage, ferrofluids, sensors, CNC machined magnetic components |
| Maghemite |
γ-Fe₂O₃ | Ferrimagnetic (moderately strong) | Similar to magnetite but with cation vacancies reducing overall magnetization | Used in recording tapes, nanoparticles, catalysts |
| Hematite |
α-Fe₂O₃ | Antiferromagnetic / Weakly ferromagnetic (under nanoscale or high temp.) | Opposite spin alignment cancels magnetism (slight net moment in small particles) | Pigments, abrasives, coatings for CNC parts |
| Wüstite |
FeO | Non-magnetic (paramagnetic) | Random spin orientations, no long-range magnetic order | Rare in nature, unstable at room temperature |
The Curie temperature is the critical point at which a ferromagnetic or ferrimagnetic material loses its permanent magnetism and becomes paramagnetic. For iron oxides (ferric oxides), this property is vital for understanding their magnetic behavior in both industrial applications and CNC machined parts where surface magnetism could affect cleaning, coating, or assembly.
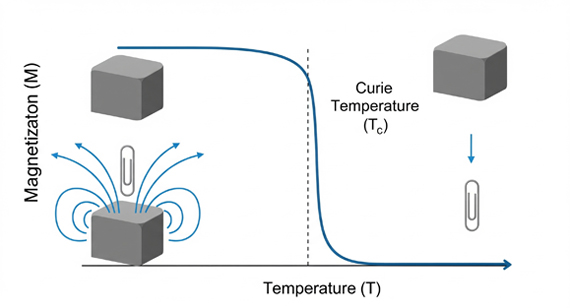
Key Curie Temperatures of Common Iron Oxides (Ferric Oxides)
Magnetite (Fe₃O₄): ~585°C
Maghemite (γ-Fe₂O₃): ~820°C
Hematite (α-Fe₂O₃): ~950 K (~677°C, Néel temperature)
Implications for CNC Machining Services
Tip: Avoid exposing magnetite-coated CNC parts to temperatures above 580°C if maintaining magnetism is essential, and always verify oxide type before high-temperature treatments.
Magnetic permeability (μ) measures how easily a material can become magnetized when exposed to an external magnetic field. For iron oxides (ferric oxides), permeability varies significantly depending on the type of oxide, crystal structure, and purity. This property is critical for CNC machined parts that will interact with magnets, sensors, or electromagnetic devices, as it influences how the part responds to stray magnetic fields and how it may attract ferrous debris.
Key Points on Magnetic Permeability
Magnetite (Fe₃O₄):
Maghemite (γ-Fe₂O₃):
Hematite (α-Fe₂O₃):
Wüstite (FeO):
Implications for CNC Machining Services
Tip: For precision CNC machined parts exposed to magnetic fields, always specify acceptable oxide types and verify their magnetic permeability to prevent functional issues in downstream assembly.
The magnetic moment of a material quantifies the strength and orientation of its magnetism at the atomic or molecular level. For iron oxides (ferric oxides), the magnetic moment arises from the unpaired electrons in iron ions (Fe²⁺ and Fe³⁺) and their alignment within the crystal lattice. This property directly determines how strongly an oxide can interact with external magnetic fields, which is crucial in applications such as CNC machined parts, sensors, magnetic coatings, and ferrofluids.
Magnetic Moment in Different Iron Oxides (Ferric Oxides)
Magnetite (Fe₃O₄):
Maghemite (γ-Fe₂O₃):
Hematite (α-Fe₂O₃):
Wüstite (FeO):
Implications for CNC Machining Services
Tip: For applications where residual magnetism is undesirable, choose oxides with low magnetic moments (hematite or carefully treated maghemite) and request demagnetization if necessary.
Permanent magnetization of iron oxide (ferric oxide) is a controlled process that aligns the magnetic domains in a way that the material retains its magnetism even after the external field is removed. This is crucial in applications such as magnetic sensors, ferrofluids, magnetic coatings, and CNC machined parts used in electronics or industrial assemblies. Understanding the step-by-step procedure ensures that iron oxide (ferric oxide) achieves the desired magnetic properties without compromising surface quality or dimensional accuracy.
1. Selecting High-Quality Iron Oxide (Ferric Oxide)
Tip: Use analytical techniques like XRD or chemical assays to confirm purity when sourcing from CNC machining factories.
Tip: Avoid abrasive treatments that can alter particle size or crystal structure, which may reduce magnetic efficiency.
3. Applying a Strong Magnetic Field
Tip: Ensure the field is uniform; uneven fields can create partial alignment and reduce permanent magnetization.
Tip: Do not exceed the Curie temperature (e.g., ~585°C for Fe₃O₄), or the material will lose magnetism.
5. Cooling
Tip: Use temperature-controlled ovens or furnaces for precision applications, especially for CNC machined components.
6. Removing the Magnetic Field
Tip: Avoid sudden removal of the field in sensitive parts, which may induce unwanted domain reorientation.
7. Testing the Magnetism of Iron Oxide (Ferric Oxide)
Tip: Document magnetic properties for quality control to ensure consistency across batches, especially in industrial or aerospace applications.
After magnetizing iron oxide (ferric oxide), it’s essential to verify the strength and stability of its magnetism, especially when used in applications like CNC machined parts, magnetic coatings, sensors, or ferrofluids. Testing ensures the material meets functional requirements and avoids issues such as unwanted magnetic interference, debris attraction, or inconsistent performance.

Methods for Testing Magnetism
Gauss Meter / Teslameter
Attraction Test
Magnetic Force Tester
Hysteresis Loop Measurement
Practical Tips for CNC Machining Applications
Tip: For industrial CNC machining projects, magnetic testing prevents problems such as metal debris attraction, sensor malfunction, or misalignment during assembly. Early detection saves both time and costs.
Iron oxide exhibits a range of magnetic behaviors depending on its type, which makes it distinct when compared to other materials. Understanding these differences is important for engineers and manufacturers, especially in CNC machining factories, where magnetic properties can affect tooling, assembly, and coating processes.
Non-Magnetic Materials
Practical Implications for CNC Machining
Selecting iron oxide (ferric oxide) for coatings or composite parts requires awareness of magnetism-related issues:
Comparing magnetic strength, permeability, and Curie temperature helps engineers choose the right material for CNC machined parts, sensors, or magnetic assemblies.
Tip: In high-precision applications, always test iron oxide (ferric oxide) layers and compare them with intended non-magnetic components to avoid contamination or interference in CNC machining processes.
Here’s a comparison table of iron oxide (ferric oxide) versus other magnetic and non-magnetic materials:
| Material |
Type |
Magnetic Behavior |
Magnetic Moment / Permeability |
Applications / Notes |
| Magnetite (Fe₃O₄) |
Iron oxide | Strongly ferrimagnetic | High; strong domain alignment | Magnetic coatings, sensors, ferrofluids, CNC machined magnetic parts |
| Maghemite (γ-Fe₂O₃) |
Iron oxide | Moderately ferrimagnetic | Moderate; lower than magnetite | Recording media, nanoparticles, magnetic coatings |
| Hematite (α-Fe₂O₃) |
Iron oxide | Weakly magnetic / antiferromagnetic | Low; mostly canceled | Pigments, abrasives, CNC machined non-magnetic coatings |
| Iron (Fe) |
Metal | Strongly ferromagnetic | Very high; easily magnetized | Structural components, electromagnets, magnetic CNC parts |
| Nickel (Ni) |
Metal | Ferromagnetic | High | Alloying, specialized magnets, CNC machined parts |
| Cobalt (Co) |
Metal | Ferromagnetic | High | Permanent magnets, magnetic coatings |
| Copper (Cu) |
Metal | Non-magnetic | Very low; paramagnetic | Electrical components, non-magnetic CNC parts |
| Aluminum (Al) |
Metal | Non-magnetic | Very low; paramagnetic | Lightweight CNC machined components |
| Titanium (Ti) |
Metal | Non-magnetic | Very low | Aerospace parts, medical CNC machined parts |
| Plastic / Ceramics / Composites |
Non-metal | Non-magnetic | Minimal | Insulating, non-magnetic parts and assemblies |
Tip: Use this table as a quick reference when deciding which materials or oxide coatings to use in CNC machining projects. Selecting the correct magnetic or non-magnetic material ensures functional performance, avoids debris attraction, and reduces interference in sensitive assemblies.
Iron oxide is a versatile material with wide-ranging applications in industrial, technological, and machining contexts. Its magnetic properties, chemical stability, and color variations make it suitable for everything from magnetic coatings to pigments in CNC machined parts. Understanding its applications ensures that engineers and machinists choose the right oxide type for both functionality and efficiency.
1. Magnetic Applications
2. Coatings and Surface Treatments
3. Pigments and Coloring
4. Catalysts and Environmental Uses
5. Electronics and Sensors
6. Medical and Biomedical Applications
Tip: When selecting iron oxide (ferric oxide) for CNC machined parts, consider both magnetic and non-magnetic properties. Using the wrong type of oxide could lead to magnetic interference, debris attraction, or coating adhesion problems, which increases project costs and reduces part reliability.
Here’s a clear table summarizing the applications of iron oxide (ferric oxide), designed for your CNC machining audience:
| Application Area |
Type of Iron Oxide (Ferric Oxide) |
Function / Purpose |
CNC Machining Relevance |
| Magnetic Devices & Sensors |
Magnetite (Fe₃O₄), Maghemite (γ-Fe₂O₃) | Provide ferrimagnetism for actuators, sensors, and magnetic assemblies | Integrated into CNC machined components for precise magnetic functionality |
| Protective Coatings |
Magnetite, Hematite | Corrosion resistance, selective magnetic properties | Applied to CNC machined parts; hematite prevents unwanted magnetism, magnetite can attract debris if not managed |
| Pigments & Coloring |
Hematite (α-Fe₂O₃), Maghemite | Red and brown pigments for paints, ceramics, plastics | Surface finishing on CNC parts for aesthetic or industrial purposes |
| Catalysts & Environmental Remediation |
Magnetite, Maghemite nanoparticles | Catalysis, water treatment, pollutant removal | Magnetic oxides allow easy separation from fluids in chemical or machining processes |
| Electronics & Data Storage |
Magnetite, Maghemite | Magnetic recording, memory, EMI shielding | CNC machined components in electronic assemblies benefit from controlled magnetic properties |
| Medical & Biomedical |
Iron oxide nanoparticles | MRI contrast agents, drug delivery, hyperthermia therapy | CNC machined carriers or supports incorporate magnetic oxides for precise placement in medical devices |
Magnetizing iron oxide (ferric oxide) involves aligning its magnetic domains to produce permanent magnetism. This process is crucial for applications like magnetic sensors, CNC machined parts, ferrofluids, and coatings. Proper magnetization ensures consistent performance, prevents debris attraction, and enables precise functionality in industrial or electronic components.
Step 1: Selecting the Right Iron Oxide (Ferric Oxide)
Tip: Use analytical methods (XRD, chemical assays) to verify oxide quality for CNC machined parts.
Step 2: Surface Preparation
Tip: Avoid abrasive treatments that could damage the crystal structure.
Step 3: Applying a Strong Magnetic Field
Tip: Ensure the field is consistent; uneven fields produce partial or weak magnetization.
Step 4: Heat Treatment (Optional)
Tip: Never exceed the Curie temperature; doing so erases magnetic alignment.
Step 5: Controlled Cooling
Tip: Use temperature-controlled ovens for CNC machined parts to avoid thermal stress or warping.
Step 6: Removing the Magnetic Field
Tip: Sudden removal of the field may partially demagnetize the material.
Step 7: Testing the Magnetism
Tip: Document all magnetization results for quality control and reproducibility in CNC machining factories.
Iron oxide is a uniquely versatile material whose magnetic properties depend heavily on its type, crystal structure, purity, and environmental factors. While magnetite (Fe₃O₄) and maghemite (γ-Fe₂O₃) exhibit strong ferrimagnetism suitable for magnetic coatings, sensors, and CNC machined parts, other forms like hematite (α-Fe₂O₃) and wüstite (FeO) are weakly magnetic or non-magnetic. Understanding these distinctions is critical for engineers, machinists, and designers to ensure functional performance, avoid interference, and maintain quality in industrial applications.
Factors such as temperature, crystal defects, alloying elements, and external magnetic fields influence the magnetism of iron oxides (ferric oxides), and controlling these factors allows for precise manipulation of magnetic properties. Practical techniques—such as careful selection of high-quality iron oxide (ferric oxide), surface treatment, applying strong magnetic fields, and controlled heating and cooling—enable the creation of permanently magnetized iron oxide (ferric oxide) for advanced applications.
From magnetic devices and coatings to pigments, catalysts, electronics, and biomedical uses, iron oxide (ferric oxide) plays a crucial role across multiple industries. By understanding its chemical composition, physical properties, magnetic moment, and interaction with other materials, CNC machining professionals can optimize their processes, select the right oxide type, and achieve reliable, high-performance parts.
Ultimately, whether designing CNC machined components, developing magnetic sensors, or applying protective coatings, knowledge of iron oxide (ferric oxide)’s magnetic properties ensures efficient manufacturing, reduced project costs, and superior material performance.
1. Can you pick up rust with a magnet?
Rust is primarily composed of iron oxides (ferric oxides), such as hematite (α-Fe₂O₃) and magnetite (Fe₃O₄). While some forms like magnetite are magnetic, common rust (mostly α-Fe₂O₃) is generally weakly magnetic or non-magnetic, so a magnet may attract only small amounts or not at all.
2. Which oxides are magnetic?
Magnetite (Fe₃O₄) and maghemite (γ-Fe₂O₃) are magnetic iron oxides (ferric oxides) due to their ferrimagnetic spinel structures. Hematite (α-Fe₂O₃) and wüstite (FeO) are generally non-magnetic or weakly magnetic.
3. Can you touch iron oxide (ferric oxide)?
Yes, iron oxide (ferric oxide) is safe to handle in most forms, such as powders or coatings. Standard precautions like gloves and masks are recommended when dealing with fine particles to avoid inhalation or contamination in CNC machining environments.
4. What types of iron are non-magnetic?
Non-magnetic types include austenitic stainless steel (e.g., 304, 316), aluminum, copper, titanium, and certain iron oxide (ferric oxide) forms like hematite. Their electron arrangements prevent permanent magnetism.
5. Does galvanized steel attract magnets?
Galvanized steel is typically magnetic because the steel substrate is ferromagnetic. However, the zinc coating itself is non-magnetic and does not affect the overall magnetism significantly.
6. Is iron oxide (ferric oxide) paramagnetic or diamagnetic?
Iron oxides can be paramagnetic or ferrimagnetic, depending on the type:
7. Is Fe₂O₃ ferromagnetic?
Fe₂O₃ exists in several forms:
So, Fe₂O₃ can be magnetic or non-magnetic depending on its crystalline form.
8. Is iron oxide (ferric oxide) a ferrite?
Some iron oxides (ferric oxides), like magnetite and maghemite, are classified as ferrites, meaning they are ferrimagnetic ceramic materials with high magnetic permeability and low electrical conductivity, widely used in magnetic applications and CNC machined parts.
9. Is rust magnetic (iron oxide)?
Rust is typically weakly magnetic or non-magnetic, depending on its composition. Magnetite-containing rust can respond to a magnet, whereas hematite-rich rust usually does not.
